As I discussed in my recent post on the “Optimal Protein Requirements for Older Cats and Cats with Hyperthyroidism,” the optimal daily protein intake for normal, young to middle-aged cats fed appears to be at least ~5.5 g/kg, whereas older cats and cats with hyperthyroidism need at least ~6.0-7.0 g/kg to prevent loss of muscle mass. If these cats already have loss of lean body mass (i.e., muscle wasting), they may require even higher amounts of daily protein to help restore lost muscle mass.
The major issue we face is to identify and feed a diet that will provide adequate amounts of protein to these older cats and cats with hyperthyroidism. Over the past couple of weeks, I’ve received a number of questions from veterinarians and concerned owners that I’d like to address. Here are the main ones:
-
How do we calculate how much protein these foods actually provide?
-
Is it even possible or practical to attempt to feed those amounts of protein?
-
What if our cats won’t eat as much protein as we would like them to ingest?
-
Why can’t we just increase the amounts of fat and carbohydrate fed to compensate for the senior (and hyperthyroid) cats’ increasing energy requirements?
-
What about older cats that have chronic renal disease? Shouldn’t these cats all be on a low protein diet?
In this post, I plan to address the first three questions. I’ll follow-up with the last two questions in separate posts because of their importance in clinical practice.
How to Calculate the Protein Content of a Food: Dry Matter Basis vs. Metabolizable Energy
Cats in the wild typically ingest diets containing > 50% of these daily calories as protein (1-3), so that may be a good goal to shoot for when selecting the composition of a diet. When analyzing a diet, I like to examine or calculate the amount of the calories each nutrient (i.e., protein, fat or carbohydrates) provides — this is called the “metabolizable energy,” abbreviated ME (4,5). This measure disregards any part of the food that does not provide any energy (kcal) such as water, ash, or fiber. It only considers the 3 nutrients that provide the needed calories and nothing else.
Let’s take an example using 5 commercial foods containing a wide range of protein content. In each of these diets, I’ve set the fat content and calories (kcal) to be equal, but I’ve varied the protein and carbohydrate amounts. Remember, when we increase or decrease the levels of protein, we must always adjust the levels of carbohydrates, fat, or both to compensate. In other words, the percentage of calories that come protein, fat, and carbohydrates must equal 100% (% protein calories + % fat calories + % carbohydrate calories = 100% of the calories ingested).
So let’s take our first hypothetical diet, which on a dry matter (DM) basis contains 65% protein, 25% fat, and 4% carbohydrate (Table 1). To convert the nutrient composition from a DM to a ME basis, we must remember that protein and carbohydrate both provide approximately 3.5 kcal/g of food, whereas fat provides much more — approximately 8.5 kcal/g of food. Thus, in this diet, the energy provided by protein is 65% times 3.5 kcal/g, or 228 kcal, and the energy provided by fat and carbohydrate are 213 kcal and 14 kcal, respectively, for a total of 454 kcal. The percentage of metabolizable energy that is provided from protein is then calculated (by dividing 228 kcal by 454 kcal and multiplying by 100), which gives us a protein content (ME) of 50%.
Doing the same calculations for diets that contain protein levels (DM basis) of 55%, 45%, 35% and 30% are also included in Table 1. As you can see, looking at nutrients on a dry matter basis “overestimates” the calories provided by protein (ME basis) by ~30%, while greatly underestimating the calories provided by fat (by ~50% in this example).
 |
Table 1. Comparison of Nutrient Content:
Dry Matter (DM) Basis vs. Metabolizable Energy (ME) Basis |
Bottom Line: When analyzing the composition of a cat food, one can look at the protein content on a DM or ME basis. In the end, it doesn’t make that much of a difference, but one must realize that the percent protein on a DM basis “overestimates” the calories provided by protein (ME basis). Cats in the wild ingest at least 50% of their calories as protein (ME). As you can see, that equates to ~65% of their diet being composed of protein on a DM basis.
Calculating the Amount of Protein that Commercial Diets Provide
Once we select our diet, we can do the calculations for protein content of a can or cup of food, as I described in detail in my post on “Optimal Protein Requirements for Older Cats and Cats with Hyperthyroidism.” Again, for this calculation, we need to know the protein content of the diet (DM basis), as well as the moisture content of the diet (generally 75% for canned food and 10% for dry food diets).
Let’s take another example by examining 5 commercial foods containing a wide range of protein content (Table 2). All of these diets are 5.5 oz cans (156 g) containing 75% moisture, so this leaves us with 39 g of food in each can on a DM basis (156 times 25% = 39 g). From the information on the label or the company’s website, we will hopefully find the protein content (DM) of each diet (note that if the protein content on a DM basis is not listed, you will have to call the company for that information). The guaranteed analysis (GA) information cannot be used for this calculation, since it listed only the minimum percentage of the crude protein contained the product and is therefore highly inaccurate.
Once the protein content (DM) is know, we multiply that value by total dry matter weight of the can. This provides us with the total protein content in each can of food (e.g., 39 g times 65% = 25.4 g of protein).
In this example, I’ve then used the weight of an average older cat (4.5 kg) to calculate the amount of protein ingested on a body weight basis (g/kg).
 |
Table 2: Calculation of Daily Protein Ingested Based on Protein Content of Food.
*Each 5.5 oz can contains 156 g of food. If moisture content of diet is 75%, that leaves 25% dry matter and 39 g of food (156 times 25% = 39 g). |
Bottom Line: As you can see in Tables 1 & 2, we need to feed a diet containing at >50% protein (DM basis) or >40% protein on a ME basis to even come close to providing the needed amounts of protein per kg.
The pet food companies may tell you that these protein levels are unnecessary and that the Association of American Feed Control Officials (AAFCO) requires that feline diets contain a minimum of 26% DM protein for maintenance (4,6). But remember: those are “minimal,” not optimal values. And most importantly, those recommendations are for normal adult cats, not hyperthyroid cats or geriatric cats prone to muscle wasting associated with sarcopenia of aging (7,8).
Is It Even Possible to Feed the Optimal Amounts of Protein to these Geriatic Cats?
The average older cat weighs 10 lbs (4.5 kg); if our goal is to provide 6.0-7.0 g of protein/kg/day, that calculates into an optimal daily protein intake of ~27-31 g. To consume those amounts of protein, that 4.5 cat would have to eat almost three 5.5-oz cans of food per day containing 30% protein (DM), almost two cans of food per day containing 45% protein (DM), or just over one can of a food containing 65% protein (DM) (Table 2).
In general, older cats tend not to eat those large amounts of food each day, unless they have uncontrolled hyperthyroidism and are polyphagic (9,11). Most older euthyroid cats (again weighing 4.5 kg) would eat only one 5.5-oz can per day, which provides only ~2.6 g/kg/day to 5.6 g/kg/day (Table 2).
So what can we do to help prevent "sarcopenia of aging" with its associated muscle wasting in these older cats? We want to feed an energy dense food that contains a highly digestible, high-quality source of protein.
Feeding an Energy-Dense Diet to Older Cats & Cats with Hyperthyroidism
What if our cats won’t eat as much protein as we would like them to ingest?
-
In addition to providing a diet with an adequate amount of protein, we need to make sure that the cat’s energy requirement is fulfilled. Most of the cat foods marketed as “Senior” diets are too low in caloric content. Remember as cats age (>12 years), their energy requirements actually increase (8, 12-14); these cats will lose weight (and muscle mass) on many of these commercial senior diets.
-
When calorie intake is inadequate to meet energy needs, body proteins are catabolized and used for energy (4). Again, feeding frequent small meals of an energy-dense, highly digestible diet that meets the senior cat’s increasing energy requirements will minimize protein degradation and avoid protein:calorie malnutrition (8, 12-14).
-
Identify diets that are palatable for the cat. Many older cats may partially lose their sense of taste or smell. Feeding a variety of different flavors or types of food helps maintain the appetite in many of these older cats.
-
Warming the food to body temperature or moistening the food may increase help to increase appetite in some cats.
-
Feeding frequent small meals of energy-dense, fresh food may help increase daily food intake.
-
Cats are solitary feeders by nature and elderly cats often do not cope well with competition and stressors. Older cats in multi-cat homes may benefit from being fed separately or being offered supplemental meals.
-
One must also identify underlying medical problems that can lead to decrease in appetite (8,9).
Feeding Highly Digestible, High Quality Protein to Geriatric Cat & Cats with Hyperthyroidism
Again, how can we help compensate for the fact that many older cats will not eat enough food to fulfill their “optimal” daily protein needs? Again, the answer may lie in the source of the protein used in the cat food diet. Feeding a highly digestible, high-quality source of protein would likely allow us to feed lower amounts of protein but still prevent loss of lean body mass and muscle wasting. Improving protein quality without increasing protein intake may help fulfill of these additional protein needs of older cats (4,15).
It is generally accepted that animal protein has a higher digestibility and is of higher quality than that of plant protein sources (16,17). Proteins of plant origin usually have a lower digestibility than animal proteins because plant fiber and the carbohydrates found in plants lower digestion, due to a reduced degradation rate of nutrients in the gut and increased bacterial activity (18).
The biological value (or quality) of a protein is a measure of that protein's ability to supply amino acids (especially the 11 essential amino acids) and to supply these amino acids in the proper proportions (4,15,17). The higher the biological value of a protein, the less would be needed in the diet to meet all of an animal’s essential amino acid requirements.
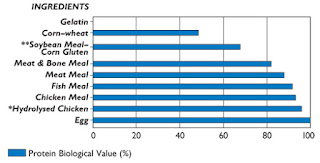 |
Figure 1: Protein Biologic Value of Common Pet Food Ingredients.
Data from reference no. 15. |
As shown in Figure 1, it is well-established that animal proteins (e.g., meat, meat by-products) have a higher biological values than vegetable proteins (e.g., corn gluten meal, soybean meal, soy protein isolate) (15-17,19). To meet a cat’s optimal daily protein needs, less protein intake would be required when its biological value is high (e.g., when animal protein is fed as the main source of protein).
What About Hill’s y/d? Will Cats Really Eat the Recommended Amounts of y/d Diet Every Day for the Rest of Their Lives ?

Again, one must realize that the amount of protein a cat ingests per day is highly dependent upon the total amount of food consumed. Eating a diet with less-than-optimal quantities of protein might be adequate if that cat ingests a large enough quantity of the food.
The recommended amounts of y/d to feed, as listed on the Hill’s website (20,21), seem reasonable for an uncontrolled hyperthyroid cat. Once euthyroid, however, will the cats continue to consume those same amounts of food? No, that's highly unlikely. In general, the amount of food hyperthyroid cats ingest decreases as euthyroidism is restored. But remember, older cats still need higher amounts of protein, even when not hyperthyroid, to maintain their muscle mass as they age.
Let’s again take our average older cat weighing 4.5 kg. For that cat to consume protein at our “optimal” amount of 6.0-7.0 g/kg/day, the cat would have to eat almost two 5.5-oz cans of canned Hill's y/d per day or ¾ cup of dry Hill’s y/d per day. Those amounts exceed the amounts listed on the Hill’s feeding guide (18,19). Again, most older euthyroid cats (again weighing 4.5 kg) would eat only one 5.5-oz can per day, which provides ~3.3 g/kg/day, about half of what they actually need.
What about the sources of protein for Hill's y/d diets? Do they include a high-quality, easily digestible source of protein? Are the ingredients of high biological value? Unfortunately, the answer to both questions is no —the ingredients present in y/d are far less than ideal for cats. In addition to the fact that y/d is a low-protein diet, much of the diet’s protein is derived from plant sources. This is especially true for the dry formulation, in which the only listed animal protein on the label is "dried egg product," and this is the fifth ingredient. In other words, this diet does not contain any meat. The primary protein source in dry y/d is corn gluten meal, commonly used in many pet foods because of its low cost.
For the canned formulation, the ingredients list is better in that the first 3 listed ingredients— liver, meat by-products, and chicken—all contain animal protein. Liver is a very nutritious organ meat and a good source of animal protein, but the daily feeding of a pet food containing liver as the first ingredient might be questioned. Do you want to feed your cats a liver diet at every meal for the rest of their lives? Would you consider that a healthy diet?
References
-
Myrcha A, Pinowski J. Weights, body composition and caloric value of post-juvenile molting European tree sparrows. Condor 1970;72:175–178.
-
Vondruska JF. The effect of a rat carcass diet on the urinary pH of the cat. Companion Animal Practice 1987;1:5-9.
-
Crissey SD, Slifka KA, Lintzenich BA. Whole body cholesterol, fat, and fatty acid concentrations of mice (Mus domesticus) used as a food source. Journal of Zoo and Wildlife Medicine 1999;30:222-227.
-
Gross KL, Yamka RM, Khoo C, et al. Macronutrients. In: Hand MS, Thatcher CD, Remillard RL, Roudebush R, Novotny, BJ (eds), Small Animal Clinical Nutrition. Mark Morris Institute. 2010; 49-105.
-
Schenck PA. Nutrients. In: Home-Prepared Dog and Cat Diets. Wiley-Blackwell. Ames IA, 2010; 23-49.
-
AAFCO. (Association of American Feed Control Officials). Official Publication, 2007.
-
Wolfe RR. Sarcopenia of aging: Implications of the age-related loss of lean body mass. Proceedings of the Nestlé Purina Companion Animal Nutrition Summit: Focus on Gerontology. St. Louis, MO. 2010, pp. 12-17.
-
Little S: Evaluation of the senior cat with weight loss, In: Little, S. (ed), The Cat: Clinical Medicine and Management. Philadelphia, Elsevier Saunders, in press.
-
The Special Needs of the Senior Cat. Information brochure, Cornell University College of Veterinary Medicine.
-
Peterson ME, Kintzer PP, Cavanagh PG, et al. Feline hyperthyroidism: pretreatment clinical and laboratory evaluation of 131 cases. Journal of the American Veterinary Medical Association 1981;183:103-110.
-
Broussard JD, Peterson ME, Fox PR. Changes in clinical and laboratory findings in cats with hyperthyroidism from 1983 to 1993. Journal of the American Veterinary Medical Association 1995;206:302-305.
-
Perez-Camargo G: Cat nutrition: What is new in the old? Compendium for Continuing Education for the Practicing Veterinarian 2004;26 (Suppl 2A):5-10.
-
Pérez-Camargo G. Feline decline in key physiological reserves: implication for mortality. Proceedings of the Nestlé Purina Companion Animal Nutrition Summit: Focus on Gerontology. St. Louis, MO. 2010, pp. 6-13.
-
Sparkes AH. Feeding old cats— An update on new nutritional therapies. Topics in Companion Animal Medicine 2011;26:37-42.
-
Lewis LD, Morris ML, Hand MS. Nutrients. In: Small Animal Clinical Nutrition III. Mark Morris Associates. Topeka, 1987; 1-25.
-
Funaba M, Matsumoto C, Matsuki K, et al. Comparison of corngluten meal and meat meal as a protein source in dry foods formulated for cats. American Journal of Veterinary Research 2002;63:1247-1251.
-
-
Murray SM, Patil AR, Fahey GC, et al. Raw and rendered animal by-products as ingredients in dog diets. Journal of Animal Science 1997; 75:2497-2505.
-
Feline Nutrition. http://maxshouse.com/feline_nutrition.htm
-
Hill's Pet Nutrition website. Prescription Diet y/d Thyroid Feline Health (Dry).
-
Hill's Pet Nutrition website. Prescription Diet y/d Thyroid Feline Health (Canned).
Źródło: endocrinevet.blogspot.com
 I started the dog on oral prednisone (0.15 mg/kg once daily) for glucocorticoid coverage. For mineralocorticoid supplementation, I administered a single dose of desoxycorticosterone pivalate (DOCP; Percorten-V, Novartis) at the recommended dose of 2.2 mg/kg intramuscularly.
I started the dog on oral prednisone (0.15 mg/kg once daily) for glucocorticoid coverage. For mineralocorticoid supplementation, I administered a single dose of desoxycorticosterone pivalate (DOCP; Percorten-V, Novartis) at the recommended dose of 2.2 mg/kg intramuscularly.